Today, more than 9 out of 10 prescriptions filled in the U.S. are for generic drugs. They’re cheaper, just as effective, and widely trusted. But this wasn’t always the case. Just 40 years ago, generics made up less than one-fifth of all prescriptions. The journey from suspicion and scarcity to dominance wasn’t smooth-it took decades of failed laws, deadly scandals, political battles, and one landmark piece of legislation that changed everything: the Hatch-Waxman Act.
The Early Days: No Standards, No Safety
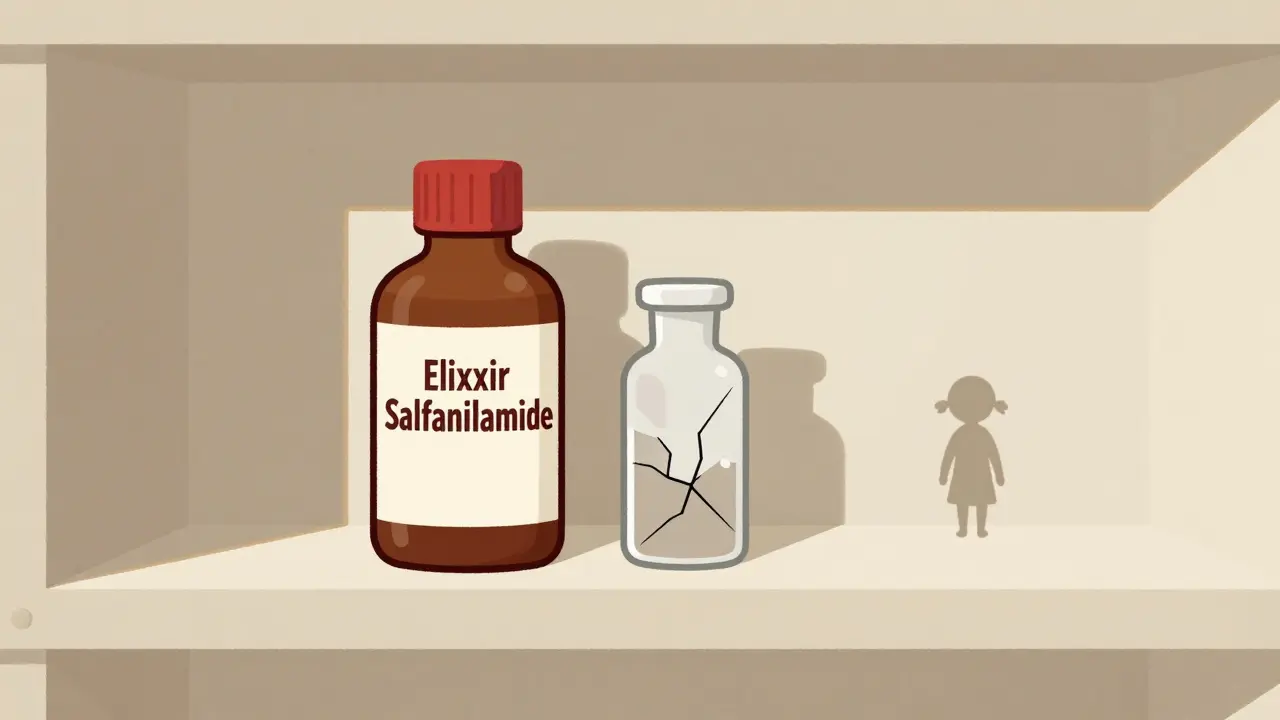
Before the 1800s, medicine in America was a wild west. Anyone could sell anything labeled as a cure. No one checked what was inside. In 1820, eleven doctors met in Washington, D.C., and created the first U.S. Pharmacopeia-a list of approved drugs and their ingredients. It was the first step toward standardization. But it wasn’t law. It was just a suggestion. By 1848, Congress passed the Drug Importation Act after too many imported medicines turned out to be fake or poisonous. Customs agents were given the power to stop bad drugs at the border. Still, there was no requirement for manufacturers to prove their products worked. That changed in 1906, after public outrage over contaminated food and drugs led President Theodore Roosevelt to sign the Federal Food and Drugs Act. For the first time, labels had to be truthful. If a drug claimed to cure cancer but didn’t contain the ingredient it promised, it was illegal. Then came the Elixir Sulfanilamide disaster in 1937. A pharmaceutical company dissolved a new antibiotic in diethylene glycol-a chemical used in antifreeze-and sold it as a liquid medicine for children. More than 100 people died, mostly kids. The public outcry was immediate. Congress acted fast. In 1938, they passed the Federal Food, Drug, and Cosmetic Act (FDCA). This law required drugmakers to prove their products were safe before selling them. It didn’t require proof they worked-just that they wouldn’t kill you. That loophole would last for another two decades.Prescription Rules and the Efficacy Gap
In 1951, the Durham-Humphrey Amendment split drugs into two categories: those you could buy over the counter and those that needed a doctor’s prescription. That system still exists today. But the big problem remained: drugs already on the market didn’t have to prove they worked. Thousands of medications, many dating back to the 1930s, were still being sold without any real evidence of effectiveness. That changed in 1962 with the Kefauver-Harris Drug Amendments. The trigger? The thalidomide tragedy in Europe, where a drug given to pregnant women caused severe birth defects. Although thalidomide wasn’t approved in the U.S., the FDA’s refusal to license it due to safety concerns made lawmakers realize how weak the system was. The new law required drugmakers to prove both safety AND effectiveness before approval. It also forced companies to submit data on all drugs sold between 1938 and 1962. Thousands of older drugs were pulled from the market because they couldn’t prove they worked. Around the same time, Medicare and Medicaid were created in 1965. Suddenly, the federal government was paying for millions of prescriptions. And it didn’t want to pay inflated prices for brand-name drugs when cheaper alternatives existed. But there were no clear rules for generics. Manufacturers didn’t know how to get approval. Doctors didn’t trust them. Pharmacies didn’t stock them. The system was broken.The Hatch-Waxman Act: The Turning Point
In 1984, Congress passed the Drug Price Competition and Patent Term Restoration Act-better known as the Hatch-Waxman Act. It was a compromise. Brand-name drug companies got extended patent life to make up for time lost during FDA review. Generic manufacturers got a clear, faster path to market. Before Hatch-Waxman, a generic company had to run full clinical trials to prove its drug worked. That cost millions and took years. The new law created the Abbreviated New Drug Application (ANDA). Generic makers no longer had to repeat expensive human trials. They only had to prove their drug was bioequivalent-meaning it released the same amount of active ingredient into the bloodstream at the same rate as the brand-name version. That cut approval time from years to months and reduced costs by 80%. The results were immediate. Generic drug use jumped from 19% of prescriptions in 1984 to over 50% by 1995. By 2022, it hit 90.5%. The savings were staggering. In 2021 alone, generics saved the U.S. healthcare system $373 billion. Over the past decade, that number exceeded $3.7 trillion.
How Generics Are Made and Approved Today
Today, the FDA’s Generic Drugs Program oversees more than 22,000 generic products and 13,000 manufacturing sites worldwide. To get approved, a generic company must submit an ANDA showing:- The active ingredient matches the brand-name drug exactly
- The dosage form, strength, and route of administration are identical
- The drug is bioequivalent-absorbed the same way in the body
- The manufacturing facility meets the same quality standards as brand-name plants
The Dark Side: Price Gouging and Shortages
Despite their success, generics aren’t perfect. Between 2013 and 2017, the price of 15% of generic drugs increased by more than 100%. Some drugs, like doxycycline or chlorpromazine, went from pennies to dollars per pill. Why? When only one or two companies make a drug, they can raise prices with little competition. In some cases, manufacturers simply stopped making low-margin drugs because it wasn’t profitable. Between 2018 and 2022, the FDA recorded 1,234 drug shortages. Two-thirds of them involved generic drugs. Many were tied to manufacturing problems, supply chain delays, or companies exiting the market. One drug, nitrofurantoin, used to treat urinary tract infections, was unavailable in many states for over a year. Patients had to switch to more expensive alternatives. Brand-name companies also found ways to block generics. Some would refuse to sell samples to generic makers, making it impossible to run bioequivalence tests. Others would pay generic companies to delay their entry into the market-a practice called “pay-for-delay.” In 2019, Congress passed the CREATES Act to stop these tactics. As of 2022, the FDA had taken 27 enforcement actions under the law.
The Future: Biosimilars and Beyond
The next frontier isn’t traditional generics-it’s biosimilars. These are copies of complex biologic drugs, like those used to treat cancer, rheumatoid arthritis, and diabetes. Unlike small-molecule generics, biosimilars are made from living cells. They’re harder to replicate and cost far more to develop. The first biosimilar was approved in the U.S. in 2015. Since then, the market has grown slowly. But with over $100 billion spent annually on biologics, the potential savings are enormous. Analysts expect biosimilars to capture 10-15% of the biologic market by 2027. That could mean billions more in savings for patients and insurers. The FDA is also working on new ways to speed up approvals for generic drugs used in emergencies, like antibiotics or vaccines. They’re testing faster review tracks and better global coordination with regulators in Europe, Canada, and Japan.Why It Matters
Generic drugs aren’t just cheaper versions of brand-name pills. They’re a cornerstone of affordable healthcare. Without them, millions of Americans couldn’t afford their prescriptions. A person on insulin might pay $300 a month for the brand name. With a generic, it’s $25. A heart medication that costs $150 per month as a brand might be $10 as a generic. The history of generics is a story of public pressure, regulatory reform, and economic necessity. It’s proof that when the system works, it can deliver massive benefits to everyday people. But it’s also a warning: without constant oversight, market forces can undermine the very thing that makes generics so valuable-competition.The next chapter will be written by regulators, manufacturers, and patients. Will we protect the system that saved trillions? Or will we let profit motives erode access to the medicines people need?
Bret Freeman
The Hatch-Waxman Act was the single most important piece of healthcare legislation in the last 50 years, and nobody talks about it. We take generics for granted like they’re just there, but behind every $5 pill is a decades-long battle against corporate greed, regulatory inertia, and medical elitism. Doctors used to look down their noses at generics like they were snake oil. Now? They prescribe them without a second thought. That’s not luck. That’s activism, litigation, and dead children forcing change.
And yet here we are in 2024, with insulin generics still priced like luxury watches because the system’s been gamed again. The same companies that fought generics in the 80s now own the patents on biosimilars and are quietly buying up small manufacturers. This isn’t progress-it’s rebranding.
We need to treat drug pricing like we treat nuclear weapons: no exceptions, no loopholes, no corporate lobbying. If a drug saves lives, it shouldn’t be a profit center. It should be a public good. Period.
Austin LeBlanc
You think this is about healthcare? It’s about control. The FDA doesn’t regulate generics because they care about you-they do it because the pharmaceutical industry pays them to regulate just enough to keep the public calm. Look at the manufacturing sites. 80% of APIs come from India and China. You think the FDA inspects every one of those factories? They inspect one and pretend the rest are clean. That’s how you get contaminated valsartan and tainted heparin. This isn’t a system-it’s a performance.
niharika hardikar
It is imperative to acknowledge that the structural evolution of generic pharmaceutical regulation in the United States represents a paradigmatic shift in public health policy architecture. The introduction of the Abbreviated New Drug Application (ANDA) framework under the Hatch-Waxman Act established a bioequivalence-centric evidentiary standard that fundamentally decoupled therapeutic efficacy from redundant clinical trials, thereby enabling cost-reflective market entry. However, the subsequent proliferation of market concentration in oligopolistic generic segments-particularly for off-patent, low-margin therapeutics-has engendered systemic vulnerabilities, including supply chain fragility and price elasticity anomalies. The Generic Drug User Fee Amendments (GDUFA) mitigated review latency but did not address the underlying anticompetitive behaviors, including pay-for-delay agreements and refusal-to-supply tactics, which continue to undermine the foundational intent of the legislation. A reconstitution of market dynamics through mandatory transparency in manufacturing ownership and tiered pricing mandates is now exigent.
John Pearce CP
Let’s be clear: America built this system. We had the science, the will, and the regulatory backbone to make generics work. Other countries? They’re still playing catch-up. But now we’re letting foreign manufacturers dictate our medicine supply-China controls the active ingredients, India handles the final product, and we sit back and call it ‘globalization.’ This isn’t free trade. This is strategic vulnerability. We outsource the pills that keep our veterans, our seniors, and our kids alive to factories that don’t answer to U.S. law. And then we wonder why there are shortages when a monsoon hits Mumbai or a factory gets shut down in Hangzhou. This isn’t capitalism. This is national security failure.
Andrea Di Candia
I’ve been on a generic heart med for five years now. It’s the same as the brand, same side effects, same results. I used to worry I was getting the ‘bad’ version. Turns out, the fear was the only thing that wasn’t real.
But I also know people who can’t afford even the generic. One friend had to choose between her insulin and rent last winter. That’s the real story here-not the science, not the laws, not the savings numbers. It’s the person standing in the pharmacy aisle holding a $25 pill and crying because their paycheck didn’t stretch far enough. We fixed the system for the pills, but not for the people who need them. Maybe the next revolution isn’t in the lab-it’s in the ballot box.
Joseph Manuel
Statistical analysis of FDA enforcement actions from 2019–2022 reveals a 67% decline in pay-for-delay settlements post-CREATES Act, yet only 12% of such cases resulted in actual market entry by generic manufacturers. This suggests that while legal deterrents have increased, structural barriers-particularly in supply chain consolidation and patent evergreening-remain intact. Furthermore, the 90.5% generic market penetration rate masks significant therapeutic class disparities: 98% of antibiotics are generic, but only 41% of psychiatric medications have approved generics. The market is not uniformly efficient. Regulatory focus must shift from volume metrics to therapeutic equity.
Andy Grace
I worked in a rural pharmacy for 12 years. I saw people come in for their cholesterol med, and the shelf was empty. No brand. No generic. Just a note: ‘Back in 3 weeks.’
One guy, 72, walked out without saying a word. I never saw him again.
Generics saved us. But we’re treating them like disposable batteries now. That’s on all of us.
Delilah Rose
It’s wild to think that something as simple as a pill can carry so much history-political, moral, economic, human. I remember when my grandma refused to take her blood pressure generic because she said, ‘The blue one always worked better.’ I didn’t understand it then. Now I do. It wasn’t about the science. It was about trust. People had been lied to for decades. The system broke their faith. And no amount of bioequivalence data could fix that right away.
But slowly, over time, as more people got their prescriptions filled without incident, as prices dropped and doctors stopped whispering about ‘cheap drugs,’ trust came back. Not because of a law, but because of consistency. Because people got better. Because they lived.
That’s what we’re protecting now. Not just the pills. The quiet promise that someone, somewhere, cares enough to make sure you can get your medicine tomorrow, and the next day, and the day after that. Even if you’re poor. Even if you’re old. Even if no one’s watching.
That’s the real win. Not the $3.7 trillion. It’s the person who didn’t have to choose between medicine and milk.
Let’s not lose that.