When you pick up a prescription, you might not think twice about whether it’s the brand-name pill or the cheaper generic. But behind that simple choice is a rigorous science designed to keep you safe. Bioequivalence isn’t just a technical term-it’s the invisible safety net that ensures a generic drug works just like its brand-name counterpart. Without it, switching to a cheaper version could mean underdosing, overdosing, or worse-side effects that weren’t there before.
What Bioequivalence Actually Means
Bioequivalence means two drugs-usually a brand-name and a generic-deliver the same amount of active ingredient into your bloodstream at the same speed. It’s not about looks, taste, or packaging. It’s about what happens inside your body. If a generic drug doesn’t match the brand’s absorption rate and total exposure, it won’t treat your condition the same way. That’s why regulators like the FDA and EMA require proof before approving any generic.
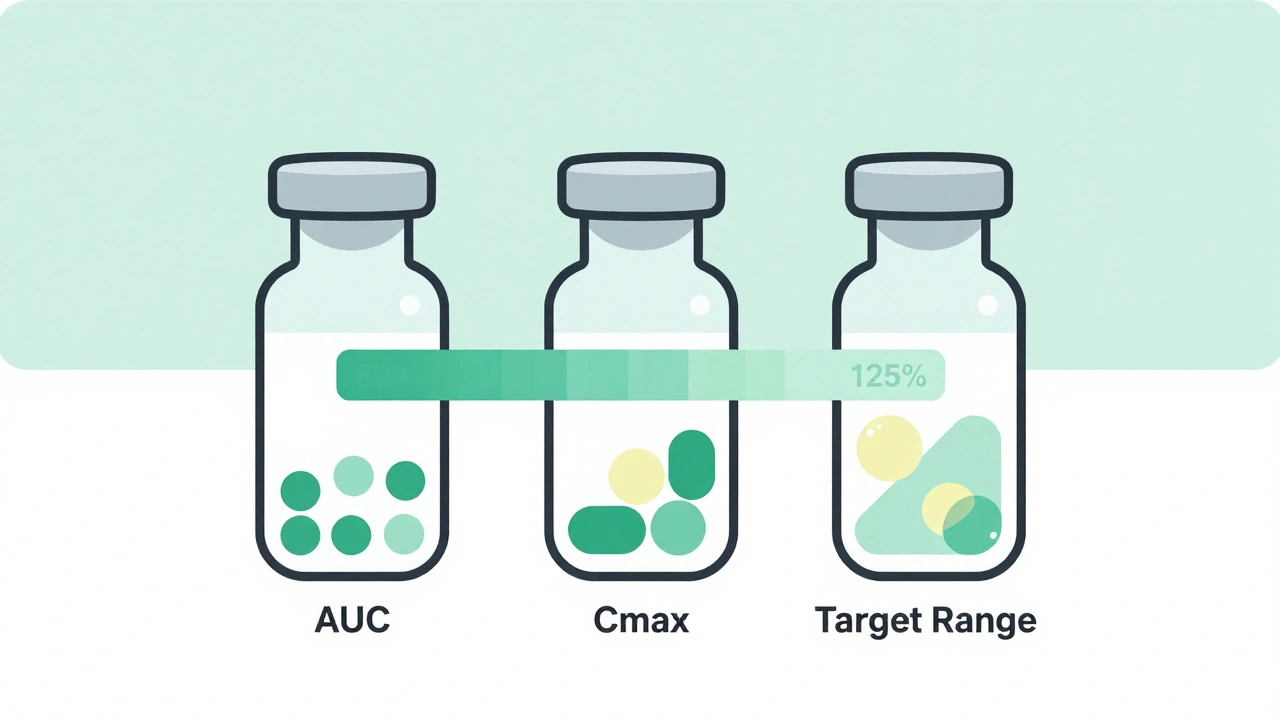
The standard? The 90% confidence interval for two key measurements-AUC (total drug exposure) and Cmax (peak concentration)-must fall between 80% and 125% of the brand-name drug. This range isn’t arbitrary. It’s based on decades of clinical data showing that within these limits, patients experience the same therapeutic effect and safety profile. For most drugs, this gap is wide enough to account for natural body differences, but tight enough to prevent dangerous variations.
Why This Matters for Your Health
Imagine you’re taking warfarin, a blood thinner with a razor-thin safety margin. A 10% drop in effectiveness could lead to a clot. A 10% increase could cause internal bleeding. That’s why drugs like warfarin, lithium, and levothyroxine fall into the “narrow therapeutic index” category. For these, regulators sometimes tighten the bioequivalence range to 90-111%. The goal? Zero tolerance for risk.
Studies show that when bioequivalence standards are followed, patient outcomes don’t change. A 2022 survey by the National Community Pharmacists Association found that 87% of patients felt generic drugs worked just as well as brand names. Even for levothyroxine-once a hot topic after switching issues in 2012-the FDA’s stricter bioequivalence rules brought stability. Today, most patients report no difference.
But what about the stories online? You’ve probably seen Reddit threads where someone says, “I switched to generic sertraline and felt awful.” These anecdotes are real to the people sharing them. But they’re not proof of failure. The FDA tracks every adverse event through its FAERS database. Between 2020 and 2023, only 0.07% of all reported adverse events involved bioequivalent generics. Brand-name drugs accounted for 2.3%. That’s not coincidence-it’s evidence that the system works.
How Bioequivalence Testing Is Done
It starts with healthy volunteers. Usually 24 to 36 people. They’re given the brand drug one day, then the generic another, after a washout period. Blood samples are taken every 15 to 30 minutes for up to 72 hours. The lab measures how much drug is in the blood at each point. That data is plugged into statistical models to see if the two products are truly equivalent.
For drugs that can’t be tested in healthy people-like cancer meds or those that cause severe side effects-studies move to patients. These are harder to run, but they’re necessary. And for drugs with active metabolites, like losartan, scientists don’t just measure the parent drug. They track the metabolite too, because that’s what actually lowers blood pressure.
Highly variable drugs-like some antibiotics or antiepileptics-are trickier. Their levels bounce around even in the same person. So regulators use a method called scaled average bioequivalence. It allows a wider range-75-133%-but only if the drug’s own variability justifies it. And even then, there’s a catch: the average difference between the two products can’t be too big. This prevents a product that’s wildly inconsistent from slipping through.
What’s Different About Biosimilars?
Not all generics are the same. Small-molecule generics-like metformin or atorvastatin-are chemically identical to the brand. Their bioequivalence is proven through blood tests. But biologics-like Humira or Enbrel-are made from living cells. They’re too complex to copy exactly. So their equivalents are called biosimilars.
Biosimilars don’t rely on bioequivalence alone. They need a full “totality of evidence” package: structural analysis, animal studies, immune response tests, and clinical trials. It’s a much longer, more expensive process. But the goal is the same: make sure the patient gets the same safety and effectiveness.
Global Rules, Same Goal
The U.S. and Europe have similar standards. Japan requires fasting studies even if the brand is taken with food. Brazil mandates a minimum set of medical checks for every volunteer. Canada, Australia, and the WHO all align on core principles. But the differences add cost and delay for manufacturers trying to sell globally.
That’s why groups like the International Pharmaceutical Regulators Programme (IPRP) are pushing for harmonization. The fewer rules that vary, the faster safe, affordable drugs reach patients. As of 2023, 134 countries have formal bioequivalence requirements-up from 89 in 2015. More nations are catching on because they see the results: lower costs, same outcomes.
Costs, Challenges, and the Future
Running a single bioequivalence study costs $1-2 million and takes 12-18 months. It needs skilled teams, advanced lab equipment, and strict compliance. Common problems? Recruiting volunteers who can follow a strict diet for fed studies. Validating tests for drugs that appear in tiny amounts. Handling high variability without skewing results.
Solutions are evolving. Replicate crossover designs help measure variability better. LC-MS/MS machines detect drugs at ultra-low levels. And now, artificial intelligence is stepping in. The FDA is using PBPK modeling-computer simulations of how drugs move through the body-to predict bioequivalence without always needing human trials. In 2022, they accepted 17 such submissions. In 2018, it was just three.
Topical creams, inhalers, and eye drops remain tough. You can’t just measure blood levels. You need to prove the drug reaches the right spot in the skin, lung, or eye. The EMA and FDA are developing new in-vitro and in-vivo tests for these. It’s slow, but progress is real.
What Patients Need to Know
You don’t need to understand pharmacokinetics to trust the system. But you should know this: bioequivalence testing isn’t a loophole. It’s the reason your generic insulin, blood pressure pill, or antidepressant works just as well as the brand. The FDA doesn’t approve generics because they’re cheaper. They approve them because they’ve proven to be just as safe and effective.
When you switch to a generic, you’re not taking a gamble. You’re benefiting from a science-backed process that’s saved the U.S. healthcare system $313 billion in 2020 alone. AARP estimates generics saved Medicare Part D $1.7 trillion from 2006 to 2020. That’s money back in people’s pockets and more access to life-saving meds.
Still, if you notice a change after switching-fatigue, mood shifts, or symptoms returning-talk to your doctor or pharmacist. It’s not always the drug. It could be a different filler, a change in your routine, or even stress. But never assume it’s the bioequivalence test that failed. The data doesn’t lie.
What’s Next?
The future of bioequivalence is smarter, faster, and more precise. AI-driven models, better analytical tools, and global alignment will keep reducing costs without cutting corners. The goal remains unchanged: ensure every patient, no matter their income, gets a medicine that works. Because safety isn’t optional. And neither is affordability.
Are generic drugs as safe as brand-name drugs?
Yes. Generic drugs must meet the same strict standards as brand-name drugs for quality, strength, purity, and performance. The only difference is cost. Bioequivalence testing proves they deliver the same active ingredient at the same rate and extent, ensuring identical safety and effectiveness. The FDA and EMA require this proof before approval.
Why do some people say generics don’t work for them?
Anecdotes about side effects or reduced effectiveness after switching are common, but they rarely reflect bioequivalence failure. The FDA tracks adverse events through FAERS and has found only 0.07% of reports involve bioequivalent generics. Most cases are due to psychological factors, changes in other medications, or unrelated health changes. If you notice a real difference, consult your provider-it’s worth investigating, but don’t assume the generic is faulty.
What’s the difference between generics and biosimilars?
Generics are exact chemical copies of small-molecule drugs like aspirin or metformin. Biosimilars are highly similar, but not identical, copies of complex biologic drugs like insulin or Humira. Generics rely on bioequivalence testing in blood. Biosimilars require a full clinical and analytical package, including immune response and functional tests, because they’re made from living cells and can’t be replicated exactly.
Do bioequivalence standards vary by country?
Core standards are very similar across major regulators like the FDA, EMA, and Health Canada. All use the 80-125% range for most drugs. But some countries have specific rules-for example, Japan requires fasting studies even if the brand is taken with food. Brazil mandates certain medical tests for all volunteers. These differences add complexity for manufacturers but don’t lower safety standards.
Can bioequivalence testing be bypassed?
Not for standard generics. Regulatory agencies require bioequivalence data as a non-negotiable part of approval. For some complex products-like topical creams or inhalers-new methods like in-vitro testing and PBPK modeling are being accepted, but they still replace clinical studies only after proving they reliably predict performance. No generic enters the market without proof of equivalence.
Every time you choose a generic, you’re not just saving money-you’re supporting a system built on science, not guesswork. Bioequivalence testing is the quiet guardian behind your prescription. And it’s working.
Chris Jahmil Ignacio
Bioequivalence is a scam cooked up by big pharma and the FDA to make you think generics are safe when they’re not. I know a guy who switched to generic levothyroxine and started having heart palpitations. They told him it was stress. Bullshit. The fillers are different. The binders are toxic. The FDA doesn’t test for those. They just check blood levels and call it a day. And now we’re all guinea pigs in a profit-driven experiment. You think they care about you? They care about the $300 billion they save every year. You’re not getting the same drug. You’re getting a cheaper version that barely passes the math. And they call it science.
Colin Mitchell
Hey, I get why people are nervous about generics - I was too at first. But I’ve been on generic sertraline for 5 years now and my anxiety’s actually better than when I was on the brand. My pharmacist even showed me the FDA’s bioequivalence data. It’s not magic, it’s math. And that math has saved millions of people from choosing between meds and groceries. If you’re having issues, talk to your doc - maybe it’s the filler, maybe it’s your sleep, maybe it’s just your body adjusting. But don’t throw out the whole system because one person had a weird reaction. The data’s solid.
Josh Bilskemper
Anyone who believes the 80-125% range is sufficient hasn’t read the actual pharmacokinetic literature. The confidence interval is statistically convenient, not biologically meaningful. For drugs with nonlinear metabolism like phenytoin or carbamazepine, even a 10% deviation can trigger toxicity or therapeutic failure. The FDA’s acceptance criteria are relics from the 1980s. Modern analytical methods can detect micro-variations in crystalline structure and dissolution profiles that the current standards ignore. And don’t get me started on the lack of real-world pharmacovigilance. You think FAERS catches everything? It catches what people bother to report. Which is less than 5% of actual adverse events. This isn’t science. It’s bureaucratic complacency dressed up in statistical jargon.
Storz Vonderheide
I work in a clinic in rural Tennessee and we switch 90% of our patients to generics. The biggest issue isn’t the meds - it’s the fear. People think if it looks different or costs less, it must be inferior. We hand out those FDA comparison charts. We show them the studies. We explain the blood level testing. And guess what? Most of them feel fine. Some even say they feel *better* because they’re not skipping doses anymore. The real win here isn’t the science - it’s access. A diabetic who can afford insulin because it’s $15 instead of $300 isn’t a statistic. They’re someone’s mom, dad, brother. Bioequivalence isn’t perfect, but it’s the best tool we’ve got to make medicine human.
dan koz
Man I read this whole thing and I’m just thinking - why do we even have brand names? In Nigeria we just get the generic and it works. No drama. No panic. No Reddit threads. If your blood pressure is down, your sugar’s stable, you’re not dying - then it’s working. Why are Americans so scared of change? The FDA is just trying to protect you from the snake oil sellers. If your generic made you feel weird, maybe you’re just anxious because you think it’s not real. I switched my aunt’s blood pressure med to generic last year. She’s still alive. Still walking. Still making jollof rice. That’s the real bioequivalence test.
Kevin Estrada
Okay but what if the generic is made in China? Like literally made in a factory where the workers are sleeping on the floor next to the pills? I saw a video. There was mold on the conveyor belt. I’m not joking. The FDA doesn’t inspect every single batch. They do spot checks. That’s it. And then they say ‘bioequivalent’ like that means anything. I’ve had my thyroid meds switch three times in two years. Each time I feel like I’m going to pass out. It’s not in my head. It’s the damn pill. And they call me crazy? I’m not crazy. I’m just the one who noticed the system’s broken. Someone’s got to say it.
Katey Korzenietz
Generics are fine unless they're not. I switched to generic citalopram and got suicidal ideation. Not a joke. Took me 3 weeks to figure out it was the pill. FAERS? They don't track individual stories. Only numbers. And numbers lie. The 0.07%? That's the tip of the iceberg. People don't report because they think they're being dramatic. Or because their doctor says 'it's all in your head'. I'm not paranoid. I'm just not stupid. And I'm not the only one.
Susan Haboustak
Let’s be clear - bioequivalence is a regulatory fiction. The 80-125% range was chosen because it’s easy to measure, not because it’s safe. Look at the drugs that have been pulled from the market after generics were approved. The 2012 levothyroxine crisis? The 2018 ranitidine contamination? The 2021 metformin NDMA scandal? All passed bioequivalence. The system isn’t designed to catch contamination, degradation, or batch variability. It’s designed to approve generics quickly so insurers can save money. You think the FDA cares about your health? They care about cost containment. And you’re the collateral damage.