When a pharmacist hands you a pill bottle labeled "generic," most people assume it’s just a cheaper version of the same medicine. But for NTI generics, that assumption can be dangerous. These are drugs where even tiny differences in how your body absorbs the medication can lead to serious harm - think blood clots, seizures, or thyroid failure. And pharmacists are sounding the alarm.
What Makes a Drug an NTI Drug?
Narrow Therapeutic Index (NTI) drugs have a very small window between a dose that works and a dose that causes harm. A 10% change in blood concentration might mean the drug does nothing - or it could kill you. That’s why drugs like warfarin, levothyroxine, phenytoin, and carbamazepine are classified as NTI. They’re not rare. They’re critical. And they’re being swapped out for cheaper generics more often than many realize.
The FDA doesn’t publish an official NTI list, but it does flag drugs in the Orange Book with codes like "B" - meaning substitution isn’t always safe. For most generics, bioequivalence is judged on a range of 80% to 125% of the brand-name drug’s absorption. For NTI drugs, the FDA recommends a tighter range: 90% to 111%. That’s a big deal. It means the generic has to perform almost identically. But even that standard isn’t foolproof.
Why Pharmacists Are Worried
According to a 2024 survey of 1,200 pharmacists by the American Society of Health-System Pharmacists (ASHP), 68% reported serious concerns about switching patients between different NTI generics. Why? Because the same drug from two different manufacturers can behave differently in the body.
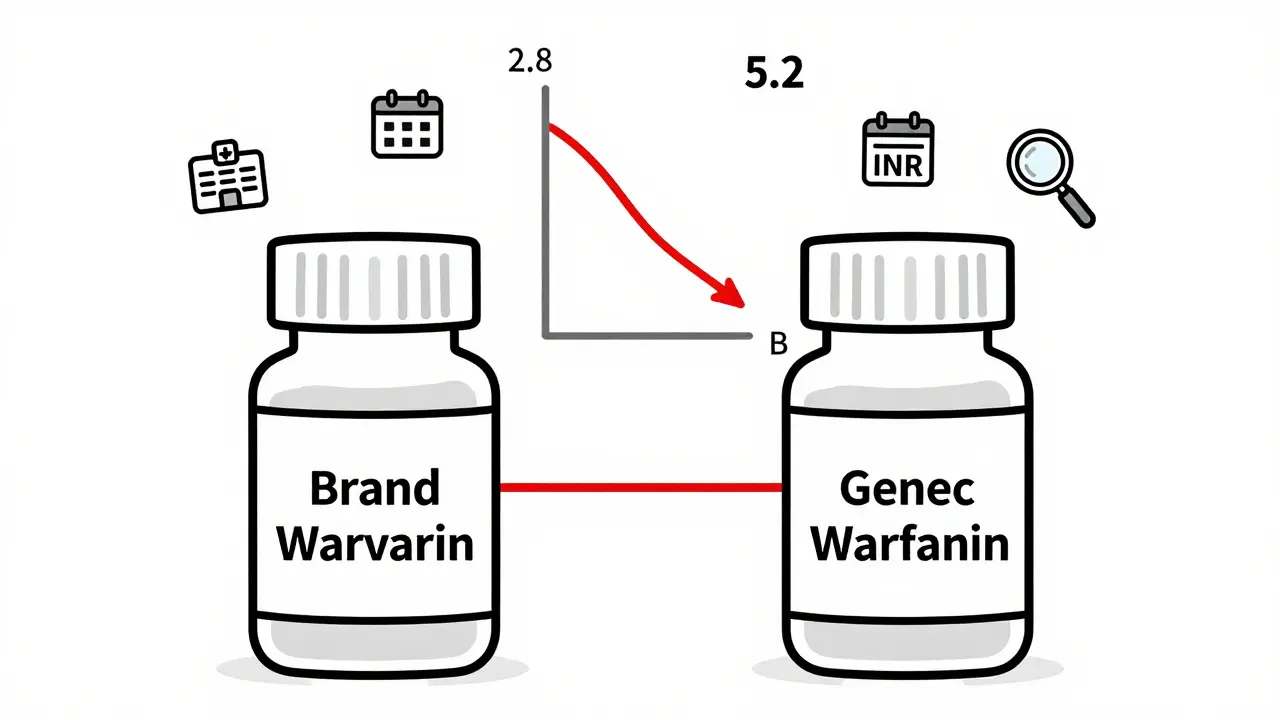
One hospital pharmacist in Wisconsin told me about a patient on warfarin who was stable for months - INR steady at 2.8. Then the pharmacy switched to a different generic version. Within five days, the INR spiked to 5.2. The patient ended up in the ER with internal bleeding. That’s not an isolated case. The FDA’s Adverse Event Reporting System recorded 1,247 incidents tied to NTI generic switches between 2020 and 2024. For non-NTI generics? Only 382.
It’s not just about one switch. It’s about constant switching. When a hospital runs out of one generic brand, they grab another. When a community pharmacy gets a new shipment, it might be from a different supplier. For NTI drugs, that’s like changing the engine in your car every time you fill up - even if the mechanic says it’s the same model.
The Cost vs. Risk Trade-Off
Generics save money. That’s the point. NTI generics cost 80-85% less than brand-name versions. For patients on long-term therapy, that’s hundreds - sometimes thousands - of dollars a year. But the savings come with hidden costs.
A 2024 study from the University of Florida found that 34% of pharmacists would never automatically substitute warfarin generics. For non-NTI drugs? Only 8%. That gap tells you everything. Pharmacists aren’t against generics. They’re against *uncontrolled* substitution.
And it’s not just about safety. It’s about trust. Patients rely on pharmacists to catch mistakes. When a pharmacist knows a patient’s INR is sensitive, they don’t just fill the script - they call the doctor. They check the label. They log the batch number. They watch for signs of instability. That extra step takes time. And it’s not always reimbursed.
State Laws Are a Patchwork
Here’s the messy part: rules change depending on where you live. As of January 2025, only 28 states have laws restricting automatic substitution of NTI drugs. In 22 states, the prescriber must explicitly say "Do Not Substitute." In six states, substitution is banned entirely for NTI drugs. In the rest? It’s up to the pharmacy - and often, the cheapest option wins.
That inconsistency creates chaos. A patient moves from California to Texas. Their levothyroxine is switched without warning. Their TSH levels go haywire. They feel tired, gain weight, get depressed. They blame themselves. The doctor blames the pharmacy. The pharmacy blames the wholesaler. No one takes responsibility.
Supply Chain Problems Make It Worse
NTI drugs are already fragile. Now, add a broken supply chain. In 2024, the FDA reported 47 NTI drug shortages - 17.4% of all drug shortages, even though NTI drugs make up only 6% of generic prescriptions. Why? Because most NTI generics are manufactured overseas. The University of Minnesota found that 80% of generics are finished in foreign countries - and for NTI drugs, that number is even higher.
When a factory in India or China has a quality issue, the FDA can’t instantly replace the supply. And when a pharmacy runs out of one generic brand, they grab another. That’s when patients get hit with unexpected changes.
Even worse, the new Medicare Drug Price Negotiation Program starting in 2026 will include three NTI drugs. Pharmacists are worried. The 21-day reimbursement delay under this program could mean pharmacies can’t afford to stock these drugs at all - especially smaller, independent ones. If they stop carrying them, patients lose access.
What Pharmacists Are Doing About It
Despite the challenges, pharmacists are stepping up. Hospitals are starting to lock in one generic manufacturer for NTI drugs and stick with it. The ASHP recommends this practice - and 63% of hospital systems now do it. That’s progress.
Pharmacy residency programs are adding NTI drug training. Eighty-one percent now include specialized modules on therapeutic drug monitoring, pharmacokinetics, and substitution risks. Community pharmacists are keeping detailed logs. They’re documenting every switch. They’re educating patients: "This is the exact brand you’ve been on. Don’t let them change it without talking to your doctor."
Some pharmacies are even using QR codes on bottles that link to batch-specific information - so patients and providers can trace exactly which version they’re taking.
What Patients Can Do
If you’re on warfarin, levothyroxine, phenytoin, or another NTI drug, here’s what you need to do:
- Ask your pharmacist: "Is this the same brand I’ve been taking?" If they say "it’s generic," ask which manufacturer.
- Request that your prescription be marked "Dispense as Written" or "Do Not Substitute."
- Know your numbers. If you’re on warfarin, track your INR. If you’re on levothyroxine, know your TSH levels. Don’t wait for your doctor to bring it up.
- Report any sudden side effects after a switch - fatigue, dizziness, irregular heartbeat, mood changes. These aren’t normal.
- Ask your doctor to write "brand necessary" if you’ve had instability with generics.
You don’t have to accept a switch just because it’s cheaper. You have the right to stability.
The Future of NTI Generics
The FDA is working on new bioequivalence standards for 12 high-priority NTI drugs, with updates expected by 2026. That’s promising. But experts like Dr. Lucinda L. Maine of the American Association of Colleges of Pharmacy say current standards still aren’t enough. "Therapeutic equivalence isn’t just about numbers on a lab report," she said in a 2024 JAMA commentary. "It’s about real people staying alive."
By 2027, 74% of healthcare systems plan to have pharmacist-led NTI drug stewardship programs - meaning pharmacists will have formal authority to manage substitutions, not just follow orders. That’s a shift toward recognizing pharmacists as clinical partners, not just fillers of scripts.
But until those systems are in place, the burden falls on you and your pharmacist. Don’t assume safety. Don’t assume consistency. Ask questions. Document changes. Advocate for stability. Your life might depend on it.

Diksha Srivastava
Wow, this is such an important topic! I’ve been on levothyroxine for years and never realized how much the generic brand could affect me. I always thought "generic = same thing" - turns out, not even close. Thanks for breaking this down so clearly. I’m going to ask my pharmacist to lock in my brand now. 💪
Sidhanth SY
Honestly, this makes total sense. I work in a pharmacy in Delhi and we’ve had patients come back with weird symptoms after switching generics. We don’t have strict rules here, so we just try to keep them on the same one if we can. It’s a mess, but at least we’re aware now. Glad someone’s talking about it.
Adarsh Uttral
so like… if ur on warfarin and they swap the generic u just get a lil more bleedin or less clotting? sounds like a gamble. why dont they just make the generics better if the stakes are so high??
Sheila Garfield
This is terrifying and I’m so glad you wrote this. I’m a nurse in London and we’ve had two patients in the last year with unexplained INR spikes after a generic switch. No one flagged it until it was too late. The system is broken. It’s not about cost - it’s about people surviving. We need mandatory tracking of batch numbers, not just a label.