Pediatric Side Effect Detection Calculator
Calculate Detection Probability
This tool estimates the likelihood of detecting rare adverse events based on your pediatric safety network size, consistent with the collaborative approach described in the article.
Detection Results
Enter values to see detection probability
When a child is given a new medication or treatment, doctors don’t always know what might go wrong. Unlike adults, kids don’t get tested as often in clinical trials. Their bodies react differently. A dose that’s safe for a teenager could cause serious side effects in a toddler. So how do we find out what’s truly safe? The answer isn’t in one hospital or one study-it’s in pediatric safety networks.
Why Traditional Research Fails Kids
For decades, most drug trials happened in adults. Then scientists assumed kids would react the same way. That didn’t work. In the 1990s, the U.S. government realized children were being left behind. Kids under 12 made up nearly 25% of the population but were included in fewer than 10% of clinical trials. Even when drugs were approved for children, the dosing was often guesswork. This gap created real danger. A child might get a medication that caused liver damage, seizures, or allergic reactions-side effects no one had seen before because no one had looked closely enough. The problem wasn’t lack of care. It was lack of data. And without data, doctors couldn’t make informed choices.How Pediatric Safety Networks Work
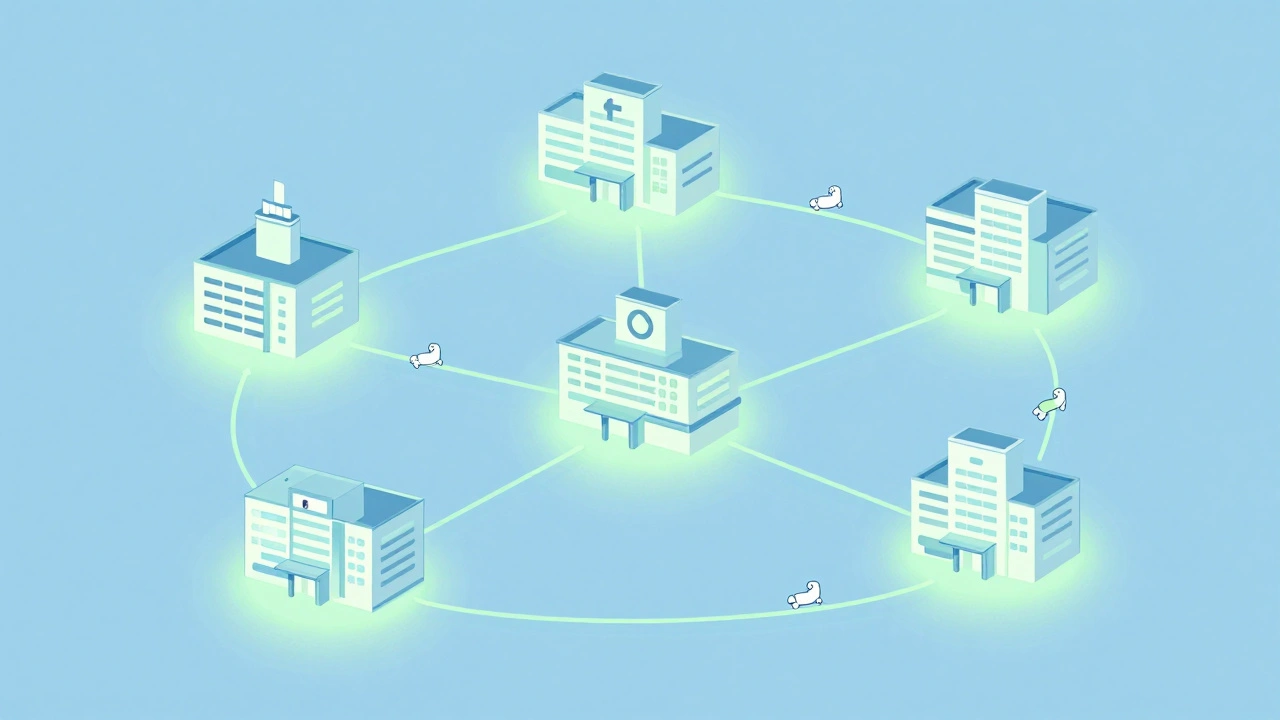
Pediatric safety networks are groups of hospitals, researchers, and public health agencies working together to track side effects in real time. They don’t wait for years to publish a study. They collect data as kids are treated, across multiple locations, and share findings immediately. One major example is the Collaborative Pediatric Critical Care Research Network (CPCCRN), a multi-site research network funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to study treatments for critically ill children and monitor adverse events in real time. Launched in 2014, it brought together seven top pediatric hospitals and a central data hub. Each site followed the same protocols. Every side effect-no matter how small-was recorded in a standardized way. The network didn’t just collect data. It had a built-in safety system: the Data and Safety Monitoring Board. This group reviewed all adverse events weekly. If a pattern emerged-a drug causing low blood pressure in 5 out of 200 kids-they could pause the trial and warn other hospitals before more children were affected.More Than Drugs: Safety Beyond the Hospital
Not all side effects come from medicine. Sometimes they come from the environment. That’s where the Child Safety Collaborative Innovation and Improvement Network (CoIIN), a state-based initiative led by the Children’s Safety Network with support from HRSA, focused on preventing injuries and tracking unintended consequences of public health programs. comes in. CoIIN didn’t study pills. It studied programs. For example, one state ran a campaign to reduce sexual violence among teens by training coaches to talk about healthy relationships. At first, they thought it was working. But when they tracked data through CoIIN’s real-time tools, they noticed something unexpected: the program didn’t reach the most at-risk kids. It was helping those already in safe environments, but not the ones who needed it most. That’s a side effect too. A well-intentioned program that missed its target. CoIIN gave them the data to fix it. They redesigned the training to include school nurses and community centers, and within a year, participation from high-risk groups jumped by 40%.
How These Networks Are Structured
These aren’t loose collaborations. They’re tightly organized systems with clear roles.- Clinical Sites: Hospitals that enroll children and report outcomes. Each must have the staff, tech, and experience to handle complex pediatric cases.
- Data Coordinating Center (DCC): The brain of the network. This team designs data forms, runs statistical analyses, and makes sure every hospital is collecting the same information. They calculate how many kids you need to enroll to detect rare side effects-something most single hospitals can’t do alone.
- Steering Committee: Made up of lead researchers from each site. They vote on which studies to run and how to handle problems.
- Data and Safety Monitoring Board: Independent experts who watch for red flags. If a side effect appears too often, they can stop a study.
What Makes These Networks Better Than Old-Style Trials
Traditional clinical trials take years. They need hundreds of volunteers, strict controls, and expensive infrastructure. They’re great for proving a drug works-but terrible at catching rare side effects. Pediatric safety networks do the opposite. They’re built for speed and scale.- They catch rare events: A side effect that happens in 1 out of 500 kids? One hospital might never see it. A network of seven hospitals treating thousands of kids each year? They’ll spot it fast.
- They adapt quickly: If a new drug causes unexpected drowsiness in toddlers, the network can alert all sites within days-not months.
- They study real-world use: Unlike lab-controlled trials, these networks track what happens when a drug is given in busy ERs, rural clinics, and under-resourced hospitals.
- They’re ethical: Some questions can’t be tested with placebo groups in children. Safety networks observe real treatments in real settings, without putting kids at extra risk.
Challenges and Limitations
They’re powerful-but not perfect. One big problem: funding. CPCCRN was funded through a five-year NIH grant that ended in 2014. No new funding cycle was announced. The network’s infrastructure didn’t vanish-it became the blueprint for later initiatives like the Pediatric Trials Network. But the momentum stalled. CoIIN ran two cohorts from 2014 to 2019. It helped 16 states and 34 teams, but after that, funding dried up. Without steady money, these networks can’t keep going. Another issue: data quality. Not every hospital uses the same electronic records. Some still rely on paper forms. The DCC had to build custom tools to make sure data from a big city hospital matched data from a small rural clinic. And then there’s the human factor. Doctors are busy. Filling out safety forms adds to their workload. One site reported that staff spent 15-20 hours a month just on data entry during peak periods. That’s a lot when you’re already short-staffed.What’s Next for Pediatric Safety Research
The future isn’t about replacing old trials. It’s about linking them. New networks are starting to connect with electronic health records (EHRs). Imagine a child gets a new asthma drug. Their hospital records it. A week later, they visit a different clinic for a cough. That clinic’s system flags: “This patient received Drug X. Watch for tremors.” That’s the goal: a national, real-time safety radar for kids. The CPCCRN and CoIIN proved it’s possible. They showed that when hospitals share data, when researchers work together, and when safety is built into every step-you don’t just find side effects. You prevent them. The next step? Making these networks permanent. Not just grant-funded projects, but part of the infrastructure of pediatric care. Because every child deserves to be protected-not just by good intentions, but by solid, shared, real-time evidence.What Parents Should Know
If your child is part of a clinical study or receives a new treatment, ask:- Is this part of a safety network?
- Are side effects being tracked across multiple hospitals?
- Will we be told if something new comes up?
amit kuamr
this is why we need more than just pharma ads telling us what's safe
kids aren't little adults and no one wants to be the first to find out the hard way
elizabeth muzichuk
I've seen this happen. My niece got a standard antibiotic dose and ended up in the ICU because no one had tracked the liver toxicity in toddlers. This system should be mandatory, not optional. Why are we still gambling with children's lives?
Debbie Naquin
The epistemological framework of distributed pharmacovigilance in pediatric populations represents a paradigmatic shift from reductionist RCTs toward emergent systems intelligence. The DCC's ontological standardization protocol enables cross-site heterogeneity to be normalized without sacrificing ecological validity. This is not merely surveillance-it's distributed cognition applied to clinical safety.
Karandeep Singh
why do we need all this fancy network stuff just to track side effects its just common sense
Mary Ngo
Let me ask you this-how do we know this data isn’t being sold to insurance companies? Who’s really controlling the Data Coordinating Center? If this is so transparent, why isn’t the public audit trail open? There’s always a hidden agenda when big pharma and government work together.
James Allen
Honestly, I’m proud of what American researchers have done here. Other countries? They’re still stuck in the 90s. We built this from scratch. And yeah, it’s messy. But we’re the only ones doing it right. Don’t let anyone tell you otherwise.
Kenny Leow
This is the kind of work that reminds me why I believe in science. 🙏
It’s not flashy, it’s not viral, but it saves lives. Kudos to the nurses typing in data at 2am and the statisticians making sense of it all. Real heroes.
Kelly Essenpreis
More bureaucracy. More forms. More paperwork for overworked doctors. We already know kids react differently. Why waste money on another network when we could just give them smaller doses?