When you pick up a prescription at the pharmacy and see a different name on the bottle than what your doctor wrote, it’s not a mistake. It’s probably a bioequivalent medication. But what does that actually mean? Is it the same drug? Will it work the same way? And why should you care?
It’s not about the label - it’s about what happens inside your body
Many people think generic drugs are just cheaper copies. That’s not wrong, but it’s incomplete. The real magic behind generics isn’t cost-cutting - it’s science. Bioequivalence is the exact, measurable standard that proves a generic drug behaves in your body just like the brand-name version. It doesn’t mean the pills look identical. It doesn’t mean the fillers or coatings are the same. It means that once swallowed, the active ingredient gets into your bloodstream at the same speed and in the same amount. The U.S. Food and Drug Administration (FDA) defines bioequivalence as the absence of a significant difference in how quickly and how much of the drug reaches your bloodstream. For most drugs, that difference must be within 80% to 125% of the brand-name version. That’s not a guess. It’s based on decades of data showing that a 20% variation in drug exposure doesn’t change how well the drug works or how safe it is for most people. Think of it like two identical cars driving the same route. One is a 2024 model, the other is a 2023 model. They have different paint jobs, different radios, maybe even different tires. But if they both hit the same speed at the same time and cover the same distance with the same fuel use - they’re functionally the same. That’s bioequivalence.How do they prove it’s the same?
To get approval, generic manufacturers don’t run new clinical trials on thousands of patients. Instead, they do bioequivalence studies - usually with 24 to 36 healthy volunteers. These people take the brand-name drug one day, then the generic another day, under tightly controlled conditions. Blood samples are taken over time to track how the drug moves through the body. Three key numbers are measured:- Cmax - the highest concentration of the drug in the blood
- tmax - how long it takes to reach that peak
- AUC - the total amount of drug absorbed over time (area under the curve)
Not all drugs are created equal - some need tighter rules
But here’s where things get tricky. For drugs with a narrow therapeutic index - where the difference between a helpful dose and a dangerous one is tiny - the rules get stricter. These include drugs like warfarin, levothyroxine, and some anti-seizure medications. For these, the FDA may require the bioequivalence range to be tighter: 90% to 111%. Why? Because even a small change in blood levels can cause problems. A 10% drop in levothyroxine might leave you feeling tired. A 10% rise could trigger heart palpitations. That’s why pharmacists often recommend sticking with the same generic manufacturer once you’ve started. If you switch from one generic brand to another, even if both are FDA-approved, the slight differences in how they dissolve or absorb can matter. A 2023 Consumer Reports survey found that dissatisfaction with generics was highest for antiepileptic drugs - 12 percentage points lower than brand-name satisfaction. Still, the FDA’s own analysis of over 2,000 generic approvals showed that 98.7% of them had drug levels within 90-110% of the brand. That’s not luck. It’s rigorous science.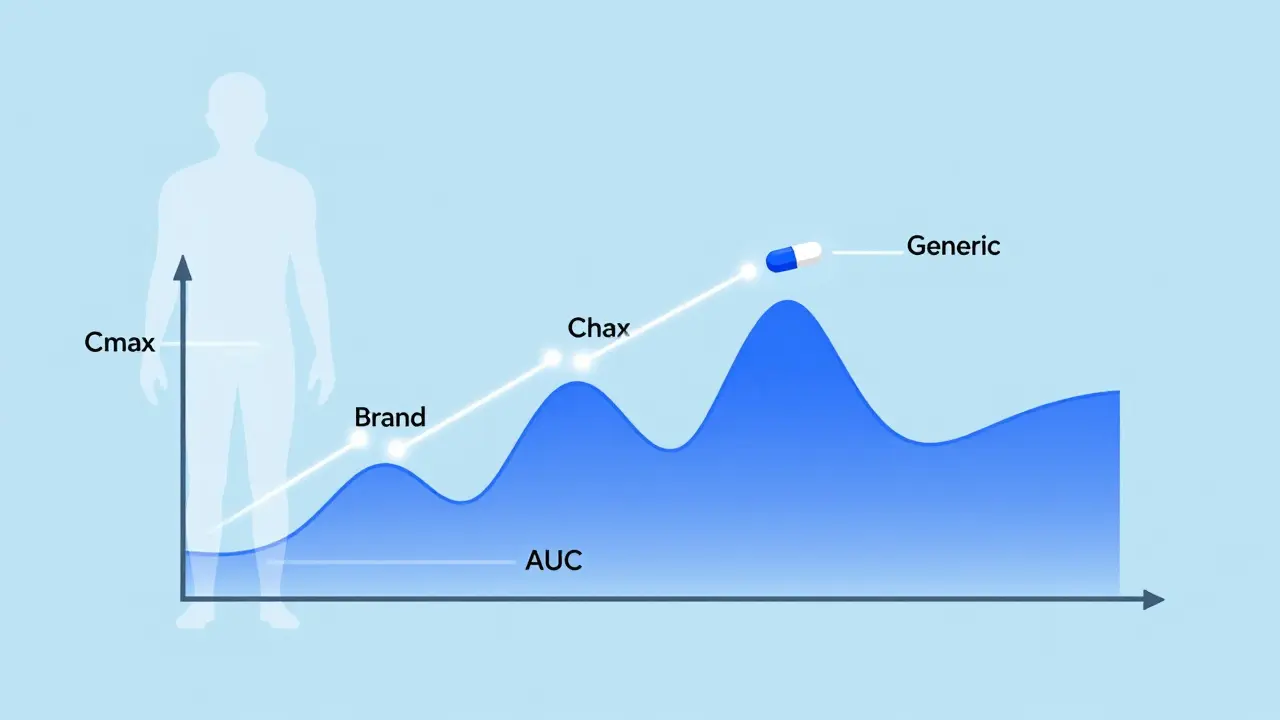
Pharmaceutical equivalence vs. therapeutic equivalence - what’s the difference?
You’ll hear these terms thrown around, but they’re not the same. - Pharmaceutical equivalence means two drugs have the same active ingredient, strength, dosage form, and route of administration. They might have different dyes, fillers, or shapes - but the medicine inside is identical. - Therapeutic equivalence means they’re not just the same on paper - they work the same in your body. That’s where bioequivalence comes in. Only drugs that are both pharmaceutical and bioequivalent get an “AB” rating in the FDA’s Orange Book, meaning they’re considered interchangeable. If a drug doesn’t have an AB rating, it doesn’t mean it’s unsafe. It just means the FDA hasn’t confirmed it behaves the same way in your bloodstream. That’s common with complex drugs like inhalers, nasal sprays, or topical creams, where absorption isn’t measured through blood tests.Are generics really safe? The data says yes
There’s a lot of fear around generics. “What if it doesn’t work?” “What if I have a bad reaction?” The numbers tell a different story. In 2022, only 0.3% of all adverse drug reports to the FDA involved generic medications - and generics make up 90% of all prescriptions filled in the U.S. That’s proportionally low. Independent pharmacists surveyed by the National Community Pharmacists Association in 2022 reported no noticeable differences in effectiveness for 87% of patients switching to generics. Even the critics admit the system works. Dr. Lawrence Yu, former deputy director of the FDA’s Office of Pharmaceutical Quality, said the 80-125% standard has been validated by real-world use over decades. The only real concerns are for a small group of drugs - and even then, the FDA has responded by tightening the rules.Why does this matter to you?
Bioequivalence isn’t just a regulatory buzzword. It’s what keeps your prescriptions affordable. Without it, generic drugs couldn’t be approved without expensive, time-consuming clinical trials. That would mean paying $300 for a month’s supply of blood pressure medicine instead of $10. The Hatch-Waxman Act of 1984 - the law that created this system - has saved the U.S. healthcare system over $2.2 trillion in the last decade. On average, each generic prescription saves patients $313 compared to the brand. But savings shouldn’t come at the cost of safety. That’s why the system exists: to ensure you get the same results, for a fraction of the price.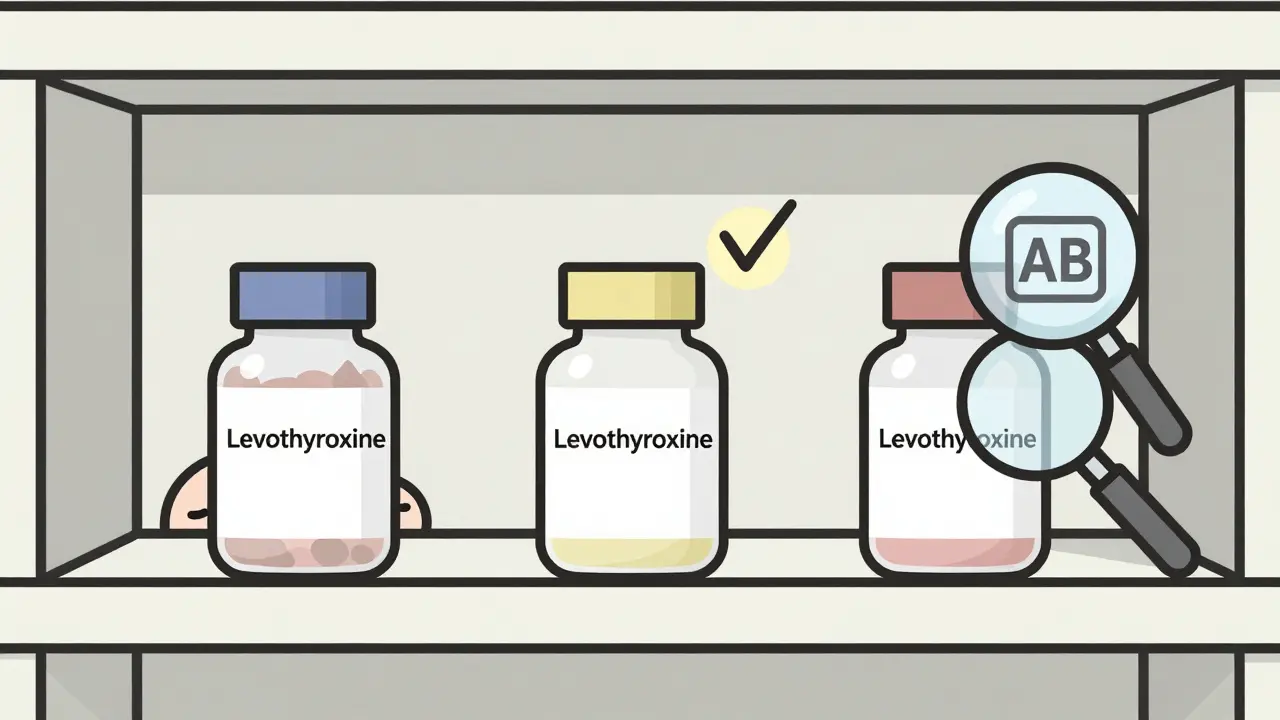
What should you do if you’re worried?
If you’ve been switched to a generic and feel different - whether it’s more side effects, less energy, or a change in how your condition is controlled - talk to your doctor or pharmacist. Don’t assume it’s “all in your head.” For drugs like levothyroxine, warfarin, or epilepsy medications, ask to stay on the same generic brand. Many states now require pharmacies to notify you before switching generics for these drugs. If you’re unsure whether your generic is approved as interchangeable, check the FDA’s Orange Book. It’s public, free, and updated monthly. Look for the “AB” rating. And remember: if a generic passes bioequivalence testing, it’s not a compromise. It’s a scientifically proven alternative.Global differences - what other countries do
The U.S. isn’t the only country using bioequivalence. The European Medicines Agency (EMA) has similar standards, but with some differences. For highly variable drugs - like some antidepressants - the EMA allows a wider range: 75% to 133%. They also require testing under both fasting and fed conditions for certain drugs, while the FDA usually picks one. That’s why a generic made in India and sold in the U.S. might not be approved in the EU - and vice versa. The science is the same, but the rules vary. For most people, this doesn’t matter. But if you’re traveling or ordering medication online, it’s worth knowing.The future of bioequivalence
The FDA is working on better ways to test complex drugs - like inhalers, patches, and injectables - where traditional blood tests don’t tell the whole story. In 2023, they released new guidance for nasal sprays and topical creams, using in vitro tests and clinical endpoint studies instead. They’ve also committed $25 million through 2027 to research new methods. Some experts are even talking about personalized bioequivalence - where the acceptable range is adjusted based on your metabolism, age, or genetics. That’s still years away, but it shows the system isn’t stuck in the past. For now, the 80-125% rule remains the gold standard. And for the vast majority of drugs, it works.Are bioequivalent medications the same as brand-name drugs?
Yes, in terms of how they work in your body. Bioequivalent medications contain the same active ingredient and deliver it at the same rate and amount as the brand-name version. They may look different or have different fillers, but they’re designed to have the same effect. The FDA requires strict testing to prove this before approval.
Why do some people say generics don’t work as well?
For most drugs, this isn’t true. But for medications with a narrow therapeutic index - like levothyroxine or anti-seizure drugs - even small changes in absorption can matter. Some patients report differences when switching between generic manufacturers, even if both are FDA-approved. That’s why pharmacists often recommend sticking with the same brand once you’ve found one that works.
How does the FDA test for bioequivalence?
The FDA requires bioequivalence studies in healthy volunteers. Blood samples are taken over time to measure three key values: Cmax (peak concentration), tmax (time to peak), and AUC (total drug exposure). The generic must stay within 80% to 125% of the brand-name drug’s results. For sensitive drugs, the range is tighter: 90% to 111%.
Can I trust a generic drug if it’s much cheaper?
Yes. The lower price comes from skipping expensive clinical trials, not from cutting corners on quality. Generic manufacturers must meet the same FDA manufacturing standards as brand-name companies. The bioequivalence requirement ensures the drug works the same way in your body - regardless of cost.
What does an AB rating mean on the FDA Orange Book?
An AB rating means the generic drug has been proven to be both pharmaceutically equivalent and bioequivalent to the brand-name drug. It’s the FDA’s official green light for substitution. If a drug doesn’t have an AB rating, it may still be safe - but it hasn’t been confirmed as interchangeable.
Carolyn Rose Meszaros
Just switched my mom to generic levothyroxine last month and she’s been feeling like a zombie 😅. Not saying it doesn’t work, but man, the difference was real. Now she’s back on the brand and her energy’s back. I get the cost thing, but some things aren’t worth risking.
Greg Robertson
Hey, I’ve been on generic metformin for 5 years now and zero issues. My A1c’s stable, no side effects. I think people freak out over nothing. The FDA’s got this locked down for most meds. Unless you’re on warfarin or seizure stuff, just chill and save your cash.
Courtney Carra
It’s funny how we treat medicine like a commodity, isn’t it? We demand consistency in our coffee, our smartphones, our Netflix algorithms - but when it comes to our bodies, we suddenly become mystics. Bioequivalence isn’t magic - it’s math. And math doesn’t lie. The 80-125% range? That’s not a loophole. It’s the boundary where biology meets statistics. We’ve been trusting numbers longer than we’ve been trusting doctors.
thomas wall
It is deeply concerning that so many Americans are willing to gamble with their health on the basis of cost savings alone. The FDA's standards may be technically sound, but they are not infallible. In Europe, we demand more rigorous testing - especially for psychotropics and endocrine agents. To suggest that a 20% variance is 'acceptable' is not science - it is corporate compromise dressed in regulatory clothing.
Paul Barnes
There’s a typo in the post: 'It doesn’t mean the pills look identical. It doesn’t mean the fillers or coatings are the same. It means that once swallowed, the active ingredient gets into your bloodstream at the same speed and in the same amount.' - missing period after 'amount.' Also, '80% to 125%' should be '80–125%' with an en dash. Minor, but it matters.
kumar kc
India makes 40% of global generics. If it works here, it works everywhere. Stop overthinking.
Thomas Varner
Okay, but have you ever tried switching between two different generics of the same drug? Like, say, from Teva to Mylan? I did - and my anxiety spiked. I didn’t change anything else. Same dosage. Same time. Just different manufacturer. And yeah, I know they’re both FDA-approved… but something’s off. The body notices things. The data doesn’t capture everything.
clifford hoang
They’re lying. The FDA doesn’t test the real stuff - they test it in healthy young men. What about elderly? Diabetics? People with liver disease? The bioequivalence studies are a farce. Big Pharma and the FDA are in bed together. They want you on generics so they can keep selling the brand-name versions overseas for triple the price. And you? You’re the guinea pig. 🧪💸
Arlene Mathison
YOU CAN DO THIS. Switching to generics isn’t settling - it’s smart. Your body is resilient. Your wallet is sacred. And if you’re worried? Talk to your pharmacist. They’re the real MVPs. Don’t let fear stop you from saving hundreds a year. You got this 💪❤️
Emily Leigh
Wait - so if two generics are both approved, why do I feel worse when I switch? Are we just supposed to ignore our own bodies because some spreadsheet says it’s ‘within range’? I mean… if my car’s engine makes a weird noise after a ‘mechanically equivalent’ part swap, I don’t just say ‘well the torque curve is within 125% so it’s fine.’